The secrets of the supernova star explosions could be hidden in dust scattered across the moon — and a team of scientists from the China Institute of Atomic Energy (CIAE) has devised a new way of unlocking those stellar death clues.
The research could help scientists obtain a clearer picture of how stars die and provide material for the next generation of stars, planets, moons, and sometimes even life — at least, when it comes to Earth.
The technique hinges on the improved detection of a rare iron isotope found in infinitesimally amounts within lunar dust. This form of iron was forged millions of years ago in the hearts of prior generations of massive stars. When these stars lost their “tug of war” battles with gravity that went on for millions (or billions) of years, thus ending their lives in supernova explosions, the isotopes would’ve been released and dispersed across the cosmos — including, scientists believe, on the moon.
Related: Peer inside remnants of an 800-year-old supernova and see a ‘zombie’ star
“Our team agreed that the only way to track historical supernova events accurately was by pushing the boundaries of what our equipment could do,” team leader and CIAE researcher Bing Guo said in a statement.
How supernovas contribute to cosmic recycling
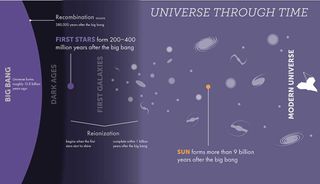
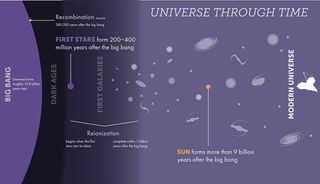
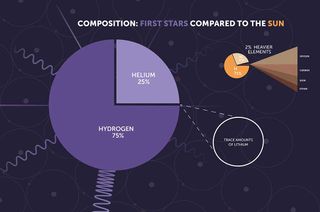
When the first generation of stars was born between 200 million and 400 million years after the Big Bang, the universe was populated mostly by hydrogen, with a sprinkle of helium. At this time, there were very few atoms of elements heavier than this, which astronomers (somewhat confusingly) call “metals.”
That means the first stars, which are paradoxically called Population III stars, were composed of hydrogen, a little helium, and barely any metals. As these stars lived, the nuclear fusion processes in their cores, by which they turned hydrogen into helium, allowed them to shine brightly in the cosmos. This fusion process also provided the outward radiation pressure that prevented the inward force of their own gravity from causing them to collapse.
That meant, however, that when hydrogen ran out in the core of these stars, the balancing act between radiation pressure and gravity ended, with the latter being the clear winner. Thus, the cores of these stars collapsed while their outer layers, where nuclear fusion was still happening, were blown away.
For stars with masses around that of the sun, this results in their stellar cores becoming white dwarf stars, surrounded by a gradually dispersing and cooling cloud of once-stellar material. That isn’t the fate of stars with are least eight times the mass of the sun, however.
When these massive stars collapse, the pressure generated in their cores triggers the nuclear fusion of helium to other heavier elements. For the most massive stars, this process repeats until the core is filled with iron, the heaviest element a star can possibly forge.
After this, a massive star’s core will collapse again, and a supernova explosion is triggered. That explosion, in turn, releases all the elements the star has generated throughout its lifetime and disperses them across the surrounding galaxy. The star then becomes a dense stellar remnant — either a neutron star or, in cases of complete gravitational collapse, a black hole.
Even that isn’t the end for the elements the star forged during its lifetime, though. These materials find their way to interstellar clouds of gas and dust, which can eventually collapse to birth stars and planets.
That is how subsequent stellar generations become increasingly “metal-rich” as time goes on. All the dispersed material is also integrated into budding planets that orbit these stars and any life forms that may exist in those worlds. Thus, when scientists say “you are star stuff,” it is more than mere lip service; it is fact.
The moon-dust-tracking team is interested in a tracer of this cosmic recycling process that isn’t an element forged during the star’s lifetime but rather a rare isotope that is created during a supernova.
Iron-60: From supernovas to the moon
Atoms are composed of three particles: in the atomic nucleus, positively charged protons and neutral neutrons reside, and “orbiting” this nucleus, negatively charged electrons zip around.
Elements are defined by the number of protons contained within their atomic nucleus. So, an atom with six protons in its nucleus is always carbon. Add another proton, and it becomes an atom of nitrogen. However, elements have more flexibility when it comes to the number of neutrons within their nucleus.
A carbon atom can have six protons and six neutrons, or it can have six protons and seven neutrons, or six protons and eight neutrons. These different variations of atoms of the same element are called “isotopes” of that element. A six-proton and six-neutron carbon atom is called “carbon-12,” while six-proton and seven-neutron carbon atoms are the carbon isotope “carbon-14.”
Some of these isotopes, especially the heavier ones, are unstable and undergo a process called radioactive decay. The period during which it takes half a given amount of a radioactive isotope to decay is called a “half-life.”
When supernovas erupt, in just a few seconds, they release as much energy as it will take the sun to radiate in billions of years. This provides the conditions needed to forge heavy radioactive isotopes. The team is looking to improve ways of hunting for a radioactive isotope of iron called “iron-60” in lunar dust.

Iron-60 has an atomic nucleus containing 26 protons and 34 neutrons, and its half-life is around 2.3 million years. While a supernova can create iron-60 in amounts equivalent to around 10 times the mass of Earth, however, the production of this isotope within the solar system is negligible. Scientists predict that, in the entire Milky Way, supernovas occur around three times every 100 years, with “nearby” star explosions being even less frequent, occurring once every million years.
Finding iron-60 on Earth, or on the moon, is a good indicator of a supernova erupting relatively close to the solar system — say, within around 100 light-years — in the recent history of our 4.6 billion-year-old planet, the research team says.
The scarcity of iron-60 and the effect of other, more common interfering elements, however, has made detecting its low abundance extremely challenging for low-sensitivity spectrometers. To combat this, Guo and colleagues made adjustments to the CIAE’s HI-13 tandem accelerator facility. This involved adding a “Wein filter,” which is a device that can be used to select charged particles traveling at specific speeds, to conduct “accelerator mass spectrometry” (AMS).
The team found AMS capable of detecting iron-60 in simulated samples with a sensitivity far beyond what can be achieved by technology typically used for these studies.
Related stories:
The CIAE team believes it’s possible to now push the detection sensitivities of their AMS system even further, a development that could majorly improve our understanding of stars that died in supernova blasts, so we could live.
“The installation of the Wien filter could be a game-changer for us,” Guo said. “Our next goal is to optimize our entire AMS system to reach even lower detection limits. Every bit of increased sensitivity opens up a universe of possibilities.”
The team’s research was published May 24 in the journal Nuclear Science and Techniques.